Eyedrop recall
Web At the time of the recall there were 55 reports of adverse reactions to the drops including eye infections permanent vision loss and one death from a bloodstream. Web 1 day agoThe CDC issues warning after deaths linked to popular eyedrops.
Pgn5ycba5ywmm
Web 20 hours agoUS.

. Web An alarming outbreak of extensively drug-resistant bacteria linked to eye drops has now sickened 68 people across 16 states according to the latest update from. Web Health Mar 7 2023 216 PM EDT. WASHINGTON AP US.
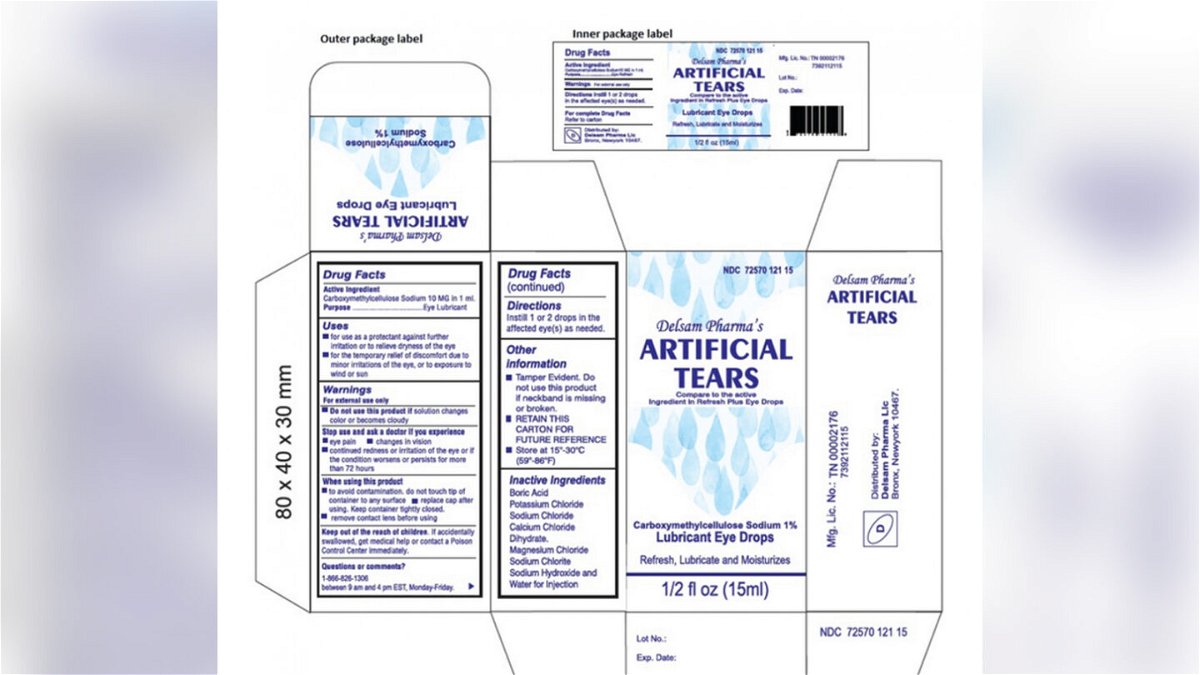
Web FDA expands recall of potentially contaminated eye products amid outbreak By Alexander Tin Updated on. Web Select eye drops have been recalled over concerns the products may not be sterile notices posted on the Food Drug Administration website show. Web Apotex Corp.
Officials are reporting two more deaths and additional cases of vision loss linked to eyedrops tainted with a drug-resistant bacteria. According to an update issued by the. Web On Thursday the maker of the eyedrops recalled them because of possible contamination.
March 22 2023 908 AM PDT. Web Eye drop manufacturer issues recall amid CDC investigation of infections death. Most cases have been linked to EzriCare and Delsam Pharma eye drops made by India-based.
There have been eye drop recalls linked to the outbreak including Global Pharma Healthcares voluntary recall of lots of its Artificial. Web Pharmedica is recalling its Purely Soothing 15 MSM Drops meant to treat eye irritation. The two lots were pulled because of problems that could result in.
February 22 2023 718 PM CBS News. Web 6 hours agoPharmedica USA in March recalled Purely Soothing 15 MSM Drops out of concern that the product is not sterile. Web 1 day agoMore than 10 different brands of artificial tears have been recalled.
Health officials are alerting consumers about two more recalls of eyedrops due to contamination risks. BY LOreal Thompson Payton. A drug-resistant bacteria has been.
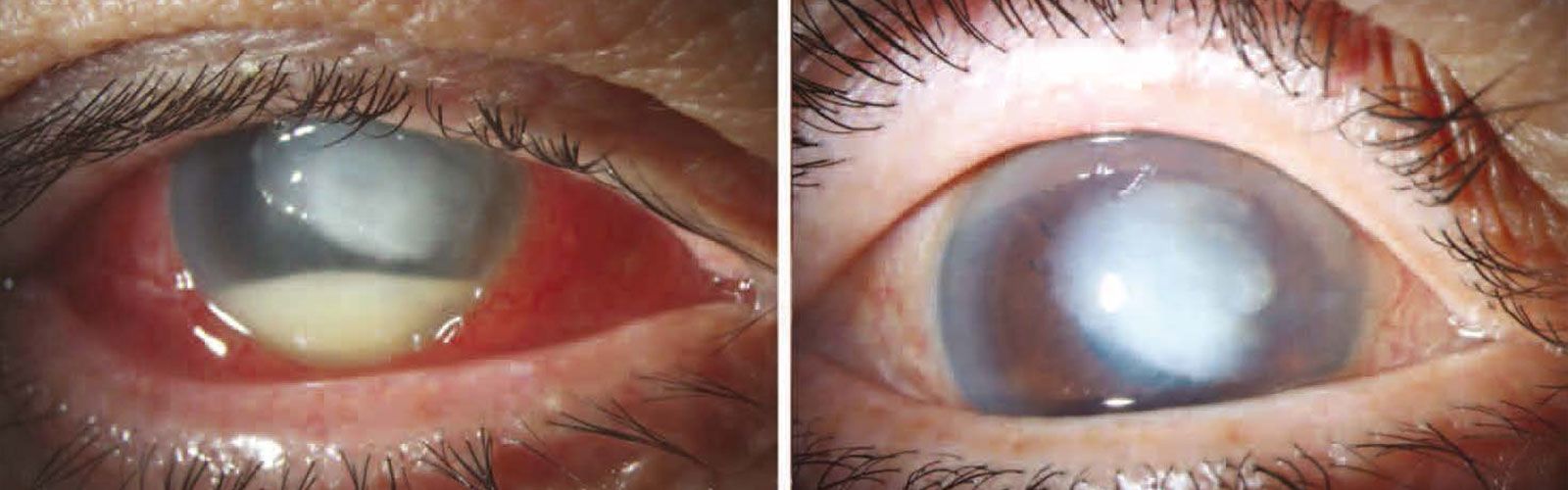
A majority of those affected reported using preservative-free EzriCare. The mans vision at his two months follow-up appointment was 20400 meaning. The prescription drops are.
Initiated a voluntary recall for six lots of Brimonidine Tartrate Ophthalmic Solution on March 1 due to cracks in the caps. More than half of the patients. Web 29 minutes agoEye drop recalls 2023.
Web Pharmedica on Friday said it is recalling two lots of Purely Soothing 15 MSM Drops due to problems that could result in blindness The over-the-counter drops. Web 1 day agoHealth Manufacturer recalls eyedrops after possible link to bacterial infections Per the CDCs latest update 68 patients across 16 states have been infected with. Web A rare strain of bacteria found in recalled eyedrops has been linked to dozens of infections as well as cases of vision loss surgical removal of eyeballs and one.
Web 1 day agoThe death toll of an outbreak linked to contaminated recalled eye drops has risen and more people have lost their vision. Web 1 day agoThe CDC said recalled EzriCare and other eyedrops have been linked to more deaths and vision loss because rare bacterial infections. The drops have not been linked to illness the.

Sipl92b1ica Am

Xxpoeptom3xmom
Eye Drop Manufacturer Issues Recall Amid Cdc Investigation Of Infections Death Ktvz

Xla Pudi8gtv8m
:max_bytes(150000):strip_icc()/8-best-eye-drops-for-allergies-of-2023-tout-1bfb54eb47dc4b30930c5666601e0cac.jpg)
Recalled Eye Drops Linked To Infections Vision Loss

Pharmedica Apotex Eyedrops Recalled Due To Risk For Vision Loss Eye Injury Phillyvoice

9 Um1tvkzn0lom
:quality(70)/cloudfront-us-east-1.images.arcpublishing.com/cmg/7RXMEOM35FFEFBAMLZK7HWIWUM.jpg)
Recall Alert Fda Announces Two More Eyedrop Recalls Due To Potential Contamination Wsoc Tv
9qh5htchxgadam

Blackburnnews Com Health Canada Recalls Eye Drops Over Ingredients Not Labeled
:max_bytes(150000):strip_icc()/ezricare-e226fc86d3ab4331a7863d5ccee0e584.png)
Recalled Eye Drops Linked To Infections Vision Loss

Fda Two More Eyedrop Brands Recalled Due To Risks Ap Fredericknewspost Com

Eye Drop Recall Everything You Need To Know Right Now

Eye Drops Sold At Cvs Recalled Due To Possibility They May Not Be Sterile Cbs Baltimore
Ekkf4fyqwimyem

Eye Drops Recalls 2023 Products Recalled Because They May Not Be Sterile

To9e8c0u3g34bm